Mission Statement
“Bringing together state-of-the-art high-throughput functional genomics and computational analysis approaches to uncover the roles of the complement system in kidney disease”
— Tilo Freiwald, MD
Team Members

Maja Gunkel

Yubing Guo

Julius Jäger
Research
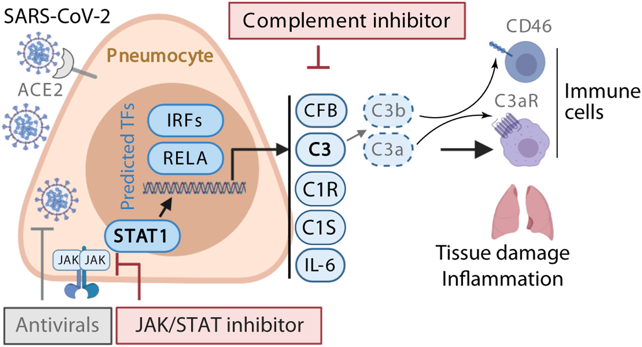
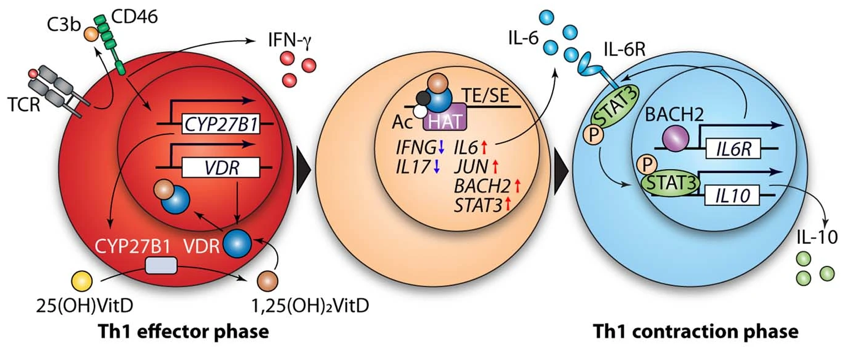
Tissue inflammation is a key determinant of kidney fibrosis and progressive loss of organ function. Defective control and deregulation of the complement pathways is a hallmark of a broad range of kidney diseases leading to fibrosis, including C3 glomerulopathy, haemolytic uraemic syndrome (HUS), lupus nephritis, and ANCA-associated necrotising glomerulonephritis. These are all diseases in which complement targeting therapies are being developed.
Complement proteins are present both within the circulation and made locally by kidney cells because these are sites inaccessible to plasma-circulating complement. Currently approved pharmaceuticals only target the complement that is present within the circulation. We believe that elucidating the local and intracellular, as opposed to systemic, complement functions will lead to a better understanding of the specific causes and consequences of complement activation in related diseases. We and others have shown that these non-canonical functions of complement fundamentally shape immune and non-immune cell function and metabolism. Improved knowledge of the role of the complement system in kidney-specific inflammation will provide a rationale to further target and personalise anti-inflammatory kidney therapeutics as well as inform the need for intracellular and local drug delivery.
Our lab routinely employs state of the art multi-dimensional single cell genomic methodologies and has computational expertise that allows us to handle big data challenges by bringing machine learning and AI to bench and bedside. Through our international partnerships we share the latest innovations and bring exchange opportunities at other top institutions to our trainees. Our role as co-founder of the medical knowledge platform AMBOSS highlights our commitment to mentorship and training in the medical field.
The Freiwald lab is committed to promotion of practices that support diversity and inclusion in clinical science.
The Freiwald lab is always looking for scientists (PhD, PostDoc) and technicians to unravel mechanism and translate the role of innate immunity′s complement system in inflammatory kidney disease. Please get in touch with your CV via

Tilo Freiwald, MD
Junior Group Leader
III. Department of Medicine, University Medical Center Hamburg-Eppendorf (UKE)
Martinistr. 52
20246 Hamburg, Germany
CV
| since 2022 | Junior group leader supported by the BMBF iSTAR Advanced Clinician Scientist program |
| since 2022 | Physician, III. Department of Medicine, University Medical Center Hamburg-Eppendorf, Prof. Dr. T. B. Huber |
|
2004 - 2011
|
Study of Medicine, University Hamburg, Germany. Clinical rotations at King’s College London, Cerrahpasa Medical Faculty Istanbul University and Stadtspital Waid Zurich
|
|
2020 - 2022- 2020
|
DFG Fellowship at Immunoregulation Section and Laboratory for Complement and Inflammation Research, National Institutes of Health, Bethesda, Maryland, USA (Dr. B. Afzali/NIDDK and Dr. C. Kemper/NHLBI) |
|
2018 - 2020
|
Postdoctoral Fellowship at Immunoregulation Section and Laboratory for Complement and Inflammation Research, National Institutes of Health, Bethesda, Maryland, USA (Dr. B. Afzali/NIDDK and Dr. C. Kemper/NHLBI) |
|
2009 - 2014
|
M.D. thesis, Institute of Immunology, University Medical Center Hamburg-Eppendorf, Prof. Dr. F. Haag |
Clinical postgraduate education:
| 2018 | Nephrology fellow at the III. Department of Medicine, University Hospital Frankurt, Germany |
| 2018 | Board Certification for Internal Medicine |
|
2017
|
Board Certification for Emergency Medicine |
|
2013 - 2018
|
Physician, III. Department of Medicine, University Hospital Frankurt, Germany |
Scientific postgraduate education:
|
2020 - 2022
|
DFG Fellowship at Immunoregulation Section and Laboratory for Complement and Inflammation Research, National Institutes of Health, Bethesda, Maryland, USA (Dr. B. Afzali/NIDDK and Dr. C. Kemper/NHLBI) |
|
2018 - 2020
|
Postdoctoral Fellowship at Immunoregulation Section and Laboratory for Complement and Inflammation Research, National Institutes of Health, Bethesda, Maryland, USA (Dr. B. Afzali/NIDDK and Dr. C. Kemper/NHLBI) |
|
2012 - 2013
|
Co-founder and Chief Technology Officer of AMBOSS GmbH |
|
since 2022 - 2020
|
Spokesperson BMBF iSTAR Clinician Scientists Hamburg |
|
2022
|
Orloff Science Award from the NIH for work on complement biology |
Selected publications
1. | C5a-licensed phagocytes drive sterilizing immunity during systemic fungal infection. |
2. | Systematic single-cell pathway analysis to characterize early T cell activation. |
3. | Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. |
4. | Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation.Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation.Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. |
5. | SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation.SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. |
6. | Renal diseases and the role of complement: Linking complement to immune effector pathways and therapeutics. |
7. | CD4+ T cell lymphopenia predicts mortality from Pneumocystis pneumonia in kidney transplant patients. |
8. | Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. |
9. | Diapedesis-Induced Integrin Signaling via LFA-1 Facilitates Tissue Immunity by Inducing Intrinsic Complement C3 Expression in Immune Cells. |
10. | Pattern recognition as a concept for multiple-choice questions in a national licensing exam. |
* Represents equal contributions as co-first or co-senior authors. |
News

Immune Response to Malaria and Chagas disease
PD Dr. Thomas Jacobs, Bernhard Nocht Institute for Tropical Medicine, „Immune Response to Infection“ ...
Martinistraße 52
Campus Research N27
20246 Hamburg Germany








